Titanium dioxide nanoparticles (nano-TiO2) and silver nanoparticles (AgNPs) are widely used in industrial products and consumer goods due to their excellent physicochemical and antimicrobial properties. As a result of their wide applications, AgNPs and nano-TiO2 may be present in the same natural aquatic systems or wastewater treatment plants.
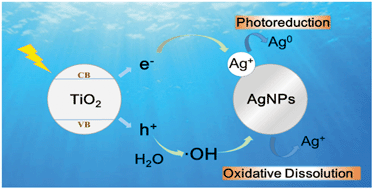
In this study, the impacts of nano-TiO2 on the transformation of AgNPs in the dark and under simulated sunlight irradiation were investigated. Under dark conditions, heteroaggregation occurred between nano-TiO2 and AgNPs. Under sunlight irradiation, nano-TiO2 promoted the dissolution of AgNPs to release Ag+ ions and the impact became more significant as the concentration of nano-TiO2 increased.
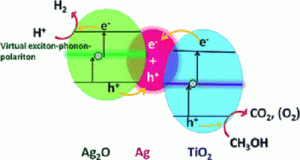
The promotion effect was attributed to the highly oxidative hydroxyl radicals generated by nano-TiO2 under sunlight irradiation. Meanwhile, some small fresh Ag0 nanoparticles formed due to the reduction of the released Ag+ ions by the photogenerated electrons on nano-TiO2. Natural organic matter, such as fulvic acid, could attenuate the promotion effect due to adsorption on the surface of nano-TiO2 and suppression of generation of hydroxyl radicals. Since Ag+ ions and small sized Ag0 nanoparticles displayed greater toxicities than large-sized AgNPs, the interaction of nano-TiO2 and AgNPs under sunlight irradiation could significantly affect the bioavailability and ecotoxicities of AgNPs.